جلد 22، شماره 1 - ( بهار 1404 )
جلد 22 شماره 1 صفحات 10-1 |
برگشت به فهرست نسخه ها
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amini-kafiabad S, Teimourpour A, Rafiee M H, Maghsudlu M, Moghtadae M. Analysis of Trends in Look-back Units Based on Post-Donation Information in Plasma Shipments for Contract Fractionation, 2018-2021. bloodj 2025; 22 (1) :1-10
URL: http://bloodjournal.ir/article-1-1570-fa.html
URL: http://bloodjournal.ir/article-1-1570-fa.html
امینی کافی آباد صدیقه، تیمورپور امیر، رفیعی محمدحسام، مقصودلو مهتاب، مقتدایی مینا. تحلیل روند واحدهای لوک بک بر مبنای اطلاعات پس از اهدای خون در محمولههای ارسالی پلاسما برای پالایش قراردادی، 1400-1397. فصلنامه پژوهشی خون. 1404; 22 (1) :1-10
استاد مرکز تحقیقات فرآورده های بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون
متن کامل [PDF 592 kb]
(722 دریافت)
| چکیده (HTML) (1776 مشاهده)
مقدمه
با تأسیس سازمان انتقال خون در سال 1353، کیتهای غربالگری برای شناسایی آنتیژن سطحی ویروس هپاتیتB (HBsAg : Hepatitis B Surface Antigen) و سیفلیس برای سرویسهای انتقال خون در جهان در دسترس بود، لذا از ابتدای تأسیس سازمان در کشور، انجام آزمایش غربالگری برای این عوامل بیماریزا انجام گردید (1).
با شناسایی ویروس هپاتیت B در ابتدای دهه 1970 میلادی، با فاصله زمانی کوتاهی کیتهای غربالگری در اختیار مراکز انتقال خون جهان قرار گرفت (2). غربالگری ویروس نقص ایمنی انسانی-1 (HIV-1 : Human Immunodeficiency Virus-1) در سال 1368 و ویروس هپاتیتC (HCV : Hepatitis C Virus) در سال 1375 در سازمان انتقال خون آغاز شد (1). با تأسیس سازمان، اهدای خون به ازای دریافت پول ممنوع بوده که نشانه دیدگاه جامع، آیندهنگر و توجه ویژه به سلامت خون است.
با ظهور HIV-1 ، سرویسهای انتقال خون سراسر جهان، به انتخاب اهداکنندگان خون توجه بیشتری کرده و پرسشنامه مشاوره اهداکنندگان خون ارتقا یافت. سازمان جهانی بهداشت (WHO : World Health Organization) برای اولین بار با مشارکت جمعیت هلال احمر و صلیب سرخ در سال 1373، راهنمای انتخاب اهداکننده خون با توجه به ویروس نقص ایمنی انسانی را منتشر کرد (4، 3). در دهه اول قرن بیستم با انتشار جزوات متعددی در زمینه ساماندهی پرسشنامه انتخاب اهداکنندگان، حفظ سلامت واحدهای اهدایی خون و بیماران دریافتکننده همراه با حفظ سلامت اهداکنندگان مورد توجه قرار گرفت (5). بهرهبرداری از نرمافزارهای معتبر، تشکیل پرونده واحد برای هر اهداکننده با ثبت تمامی اطلاعات هر نوبت اهدا، سبب ارتقا سلامت خون گردید (1).
با شناسایی HIV با هدف جلوگیری از گسترش عفونت با آن از طریق بیمارانی که خون و فرآورده قبل از غربالگری برای HIV دریافت کرده بودند، در کشورهایی مانند کانادا، آمریکا و انگلیس لوک بک (Lookback) اجرایی شد. فرآوردههای تولیدی از خون اهداکنندگان مثبـت بـرایHIV قبـل از سـال 1980، شناسایــی شـده و
اطلاعات بیماران دریافتکننده استخراج گردید. این بیماران جهت انجام آزمایش برای تشخیص عفونت با HIV فراخوان شدند (8-6). علاوه بر HIV با کشف و شناسایی HCV ، لوک بک برای این ویروس نیز در کشورهای مختلفی مانند کانادا، دانمارک و آمریکا آغاز شد (11-9). در این فرآیند بیمارانی مانند کودکان، بیماران هموفیلی و بیماران مورد جراحیهای ویژه مانند جراحی قلب مورد آزمایش قرار میگرفتند تا در صورت ابتلا به عفونت با آموزش و درمانهای حمایتی از توسعه بیماری در جامعه جلوگیری شود (13، 12). این فرآیند را لوک بک (Lookback) نامگذاری و در استانداردهای انتقال خون مد نظر قرار دادند (15، 14).
در طی سالهای بعد لوک بک توسعه یافت و شامل واحدهای اهدایی بر اساس نتایج مثبت آزمایشهای غربالگری ویروسهای منتقله از راه خون یا اطلاعات پس از اهدا (Post donation information)، در رابطه با واحدهای اهدای قبلی اهداکننده بود. احتمال این که اهداکننده در دوره کمون بوده و با آزمایشهای غربالگری قابل شناسایی نباشد، بسیار اندک است، به همین دلیل انتقال عفونت پس از تزریق خون و فرآوردهها که به عنوان خطر باقیمانده (Residual Risk) نامیده میشود، ناچیز است. در این فرآیند واحدهای لوک بک امحا، قرنطینه، آزمایش واحدهای در دسترس و اطلاعرسانی به دریافتکنندگان انجام میشود (16، 8).
اطلاعات پس از اهدا (PDI) در چندین گروه قرار میگیرند که مهمترین اطلاعاتی هستند که توسط خود اهداکنندگان اعلام میشود که آن را به دو زیر گروه میتوان دستهبندی کرد. در یک دسته اهداکننده خود مطلع بوده و اعلام نکرده و اگر در حین مشاوره به پزشک اعلام میگردید، اهداکننده از اهدای خون توسط پزشک معاف میشد. گروه دیگر اهداکننده در زمان اهدا از عامل خطرساز مطلع نبوده و با اطلاع پس از اهدای خون، مرکز انتقال خون را مطلع میسازد و به عنوان Call-back شناخته میشود. عملکرد اهداکنندگان در این گروه انعکاس رفتار مسئولانه است هر چند خطر بالقوه برای سلامت خون باید مد نظر باشد. در انتقال خون و سلامت آن عدم افشای موارد منجر به معافیت در فرآیند انتخاب اهداکننده مهم است. شناسایی علت یا علل عدم اعلام رفتارهای پرخطر به پزشک و انجام اقدامات اصلاحی برای آنها میتواند منجر به ارتقا و بهبود فرآیند انتخاب اهداکنندگان و افزایش سلامت خون گردد (17).
با شروع پالایش قراردادی پلاسما در سال 1385 در کشور، فرآیند لوک بک با رویکرد نتایج آزمایشهای غربالگری استقرار یافت (18). متعاقباً "اطلاعات پس از اهدا" نیز اضافه شد. زیرگروه دیگر احتمال بیماری کروتسفلد ژاکوب (CJD : Creutzfeldt-Jakob)، را میتوان نام برد (20، 19، 13).
عواملی که در گروه رفتارهای پرخطر با عنوان اطلاعات پس از اهدا (PDI) بوده و منجر به لوک بک میشود، در جدول ذکر شده است (21، 15) (جدول 1). در این فرآیند امحا فرآوردههای خون از جمله پلاسما سبب افزایش ضایعات آن میشود.
ویلیامز و همکاران در سال 1997 با استفاده از پرسشنامه که در آن از رفتارهای پرخطر که میتواند سبب معافیت در آخرین اهدای خون اهداکنندگان شود، طرح پژوهشی اجرا کردند. بر اساس اطلاعات استخراجی از پرسشنامهها، 9/1% اهداکنندگان در زمان اهدای خون رفتار پرخطر داشته و در صورت اعلام آن از اهدای خون معاف میشدند. در این بررسی در 4/0% موارد در بازه زمانی 3 ماه قبل از اهدای خون رفتار پرخطر رخ داده بود (22). این مطالعه نیز دلیلی بر ضرورت انجام لوک بک به علت اطلاعات پس از اهدا را تایید میکند.
در این مطالعه واحدهای لوک بک به علت رفتار پرخطر در بازه زمانی 1400-1397 در محمولههای ارسالی پلاسما برای پالایش قراردادی که از مراکز انتقال خون به سردخانههای ستاد مرکزی سازمان حمل شده بود، از نظر تعداد و عوامل خطرساز تحلیل شدند تا در هنگام انتخاب اهداکنندگان عوامل خطرساز منجر به لوک بک مورد توجه بیشتر قرار گیرد.
مواد و روشها
در ایـن مطالعـه مقطعـی، آمار اهدای خون و معافیت از
اهدا از نرمافزار یکپارچه معتبر انتقال خون، نگاره به تفکیک سالهای مطالعه دریافت گردید. میزان ارسال پلاسما با استفاده از مکاتبات گواهی سلامت که سازمان انتقال خون به سازمان غذا و دارو و نمایندگان شرکتهای پالایشگر انجام میدهد، استخراج شد.
مراکز انتقال خون مورد مطالعه:
پالایش قراردادی پلاسما در سال 1385 با 15 پایگاه انتقال خون آغاز شد. در طی سالهای پس از آن با افزایش تعداد مراکزی که توسط حوزه تضمین کیفیت شرکتهای پالایشگر و نهادهای دولتی کشورهای شرکتهای پالایشگر بازرسی شدند، در سال 1400 به 28 مرکز افزایش یافت. در طی بازه زمانی مورد مطالعه 5 پایگاه انتقال خون بازرسی و مجوز ارسال پلاسما دریافت کردند.
جمعآوری اطلاعات لوک بک متعاقب اطلاعات پس از اهدا:
فرم لوک بک کاملاً خودکار توسط نرمافزار نگاره در ادارات کل آماده و چاپ شده، سپس توسط کارشناسانی که آموزشهای لازم را دریافت کردهاند، بررسی میگردد. در مرحله بعد توسط مدیریت/معاون اداره کل تأیید شده و در مرحله بعد به ستاد مرکزی سازمان انتقال خون ارسال میشود. اطلاعات این مطالعه از فرمهای لوک بک ارسالی از ادارات کل توسط کارشناسان ستاد استخراج گردید (18).
اطلاعاتی که در فرمهای لوک بک ثبت شده و در این مطالعه مورد استفاده قرار گرفت شامل شماره اهدا، تاریخ رخداد پرخطر، نوع رفتار پرخطر، تاریخ آخرین اهدا با نتایج آزمایشهای غربالگری منفی، تاریخ معافیت و نوع معافیت (دائمی یا موقت) و رفتار پرخطر طبق جدول یک بود.
دستهبندی رفتارهای پرخطر و نحوه شناسایی واحدهای لوک بک:
در موافقتنامه کیفی با شرکتهای پالایشگر و در سرویسهای انتقال خون کشورها، رفتارهای پرخطری که در حیطه لوک بک قرار میگیرد و بازههای زمانی که باید برگشت به عقب نمود، مشخص و تعیین شده و در جدول 1 ذکر شده است (21، 15). اگر تاریخ رفتار پرخطر در هنگام مصاحبه با اهداکننده توسط وی اعلام شود، تاریخ آخرین اهدای خون که در آزمایشهای غربالگری منفی گزارش شده شناسایی میگردد و از این تاریخ به مدت 183 روز تا رسیدن به تاریخ رفتار خطرساز، کلیه واحدهای اهدایی لوک بک میشود. این بازه زمانی حداکثر 6 ماه است و اگر رفتار پرخطر با واحد اهدایی منفی کمتر از 6 ماه فاصله داشته باشد، این بازه از 6 ماه کمتر خواهد بود. اگر اهداکننده تاریخ رفتار پر خطر را فراموش کرده باشد کلیه واحدهای پلاسما در بازه زمانی 6 ماه قبل از معافیت لوک بک خواهد شد.
روش تجزیه و تحلیل دادهها:
آمارهای توصیفی شامل میانگین و انحراف معیار به همراه فراوانی و درصد فراوانی گزارش شده است. به منظور بررسی روند تغییرات نسبت واحد لوک بک به تعداد واحد خون و واحد پلاسمای ارسالی از آزمون کوکران- آرمیتاژ استفاده شده است (23). از شاخص خطر نسبی به عنوان اندازه اثر برای مقایسه روند تغییرات لوک بک در طی زمان استفاده و گزارش شده است. در این مطالعه سطح معناداری 05/0 در نظر گرفته شده است. تمام محاسبات آماری توسط نرم افزار R و مقادیر خطر نسبی و فواصل اطمینان 95% آن به وسیله پکیج epitools محاسبه گردیده است.

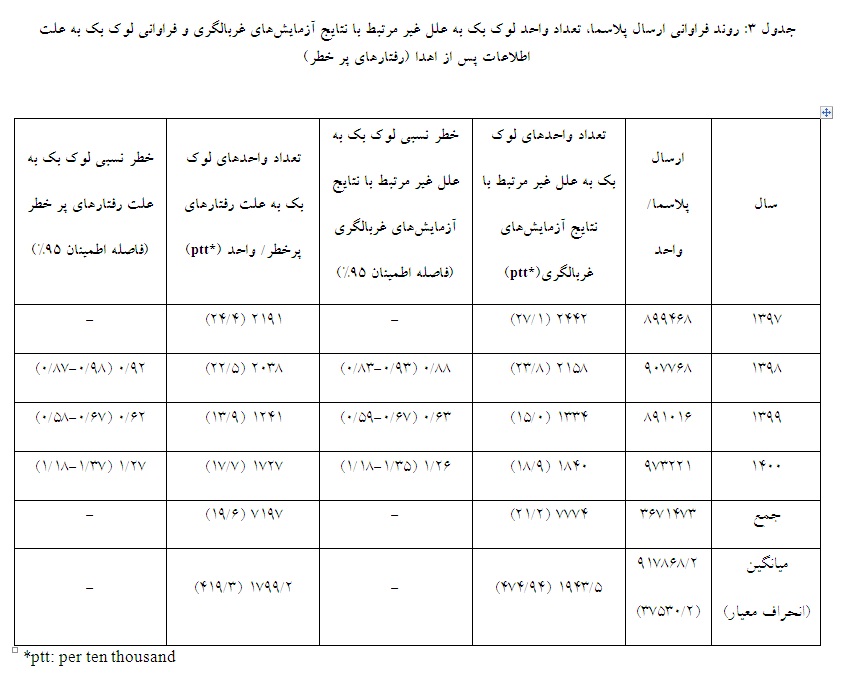
مقادیر خطر نسبی لوک بک غیر از موارد لوک بک به علت نتایج آزمایشهای غربالگری تکرارپذیر در هر سال در مقایسه با سال قبل در جدول 3 گزارش شده است. روند کاهشی در میزان تعداد واحد لوک بک به علت اطلاعات پس از اهدا مبتنی بر رفتارهای پر خطر در طی زمان از 1397 تا 1400 نسبت به میزان واحد پلاسمای ارسالی مشاهده میشود (001/0 p<)، در سال اول مطالعه نسبت واحد لوک بک به علت رفتارهای پر خطر 4/24 در ده هزار واحد پلاسمای ارسالی است که در سال آخر مطالعه به 7/17 در ده هزار واحد پلاسمای ارسالی کاهش یافته است (001/0 p< ؛ 78/0-68/0 :95%CI ؛ 73/0 RR:)، مقادیر خطر نسبی واحد لوک بک به علت رفتارهای پر خطر در هر سال در مقایسه با سال قبل و اطلاعات مرتبط با فراوانی تعداد واحد پلاسمای ارسالی و تعداد واحد لوک بک به علت رفتارهای پرخطر در هر سال در جدول 3 گزارش شده است.
طبق اطلاعات جدول 3، با توجه به اطلاعات ستونهای تعداد واحدهای لوک بک به علل غیر مرتبط با نتایج آزمایشهای غربالگری و تعداد واحدهای لوک بک به علت رفتارهای پرخطر، تعداد واحدهای لوک بک غیر مرتبط با اطلاعات پس از اهدا و نتایج آزمایشهای غربالگری (مانند CJD و سایر علل) نیز سیر نزولی داشته و از 7/2 به 2/1 به ازای هر ده هزار واحد، معادل 6/1 واحد در هر ده هزار واحد پلاسما ارسالی کاهش نشان میدهد.
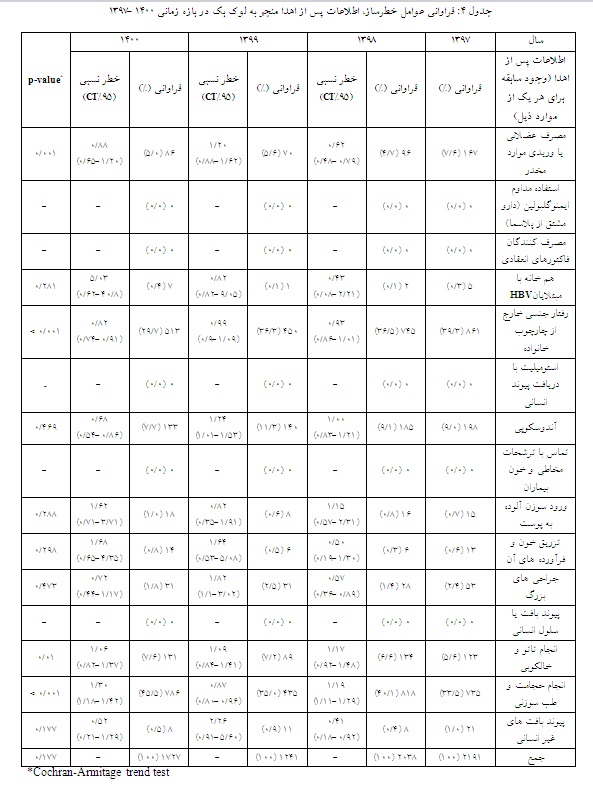
اطلاعات پس از اهدا، یا رفتارهای پرخطر که منجر به لوک بک شدند در 15 گروه طبق موافقت نامه کیفی با پالایشگرها قرار گرفتند. در 5 گروه در بازه زمانی مورد مطالعه نمونهای گزارش نشده است. در بین 10 گروه دیگر بیشترین فراوانی در گروههای رفتار جنسی خارج از چارچوب خانواده، حجامت و طب سوزنی، انجام تاتو و خالکوبی، آندوسکوپی و مصرف مواد مخدر تزریقی با فراوانی 6/5% تا 3/39% گزارش شد. از این 5 گروه، 4 گروه شامل رفتار جنسی خارج از چارچوب خانواده (001/0 p<)، حجامت و طب سوزنی (001/0 p<)، انجام تاتو و خالکوبی (01/0 p=) و مصرف مواد مخدر تزریقــی
(001/0 p=) در بازه زمانی مطالعه با یک روند معنادار گزارش شده است.
بحث
در طی سالهای 1400-1397 بخشی از واحدهای لوک بک که مرتبط با انتخاب اهداکننده و رفتارهای پرخطر است، روند کاهشی نشان میدهد. شاید به نظر برسد این کاهش به نحوه انتخاب اهداکننده و بهبود آن مرتبط باشد اما از سوی دیگر شاهد روند کاهشی تعداد معافیت هستیم که با توجه به پاندمی کووید-19 و افزایش حداقل 4 سؤال به پرسشنامه اهداکنندگان برای جلوگیری از اهدای خون در داوطلبانی که احتمال انتقال کووید 19 را دارند، نیاز به توجه بیشتری در تحلیل یافتهها دارد (24). در سال 1399 کاهش آمار اهدای خون به دنبال کاهش داوطلبان برای اهدای خون مشاهده میگردد. دربازه زمانی این مطالعه، نوسان در فراوانی موارد مثبت HBV ، HCVوHIV در کشور گزارش شده است از جمله فراوانی HBV و HCV در سال 2020 نسبت به سال 2019 در جمعیت اهداکنندگان افزایش یافته و این افزایش معنادار گزارش شده است (27-25). با توجه به موارد فوق، اعلام نظر برای بهبود و ارتقای فرآیند انتخاب اهداکننده همانند سایر کشورها در این بازه زمانی که مصادف با پاندمی کووید بوده، با چالشهای جدی روبرو است (29-27).
در سال 1399به عنوان اولین سال پاندمی کووید 19 که برای جامعه جهانی غیرمترقبه و بدون آمادگیهای لازم برای مراکز انتقال خون در برخورد با این پاندمی بود، کمترین تعداد لوک بک به علت رفتارهای پرخطر، 1392 مورد به ازای هر 10000 واحد پلاسما ارسالی، گزارش شده است. اما در سال 1400 این تعداد به 1774 واحد به ازای هر 10000 واحد پلاسما ارسالی برای پالایش قراردادی رسیده است. این تغییرات همراه با تغییر میزان معافیت از اهدای خون در سال 1400 همانند سایر کشورهای جهان میتواند متأثر از پاندمی کووید 19 باشد (32-30).
از عوامل خطر ساز در لوک بک به دنبال اطلاعات پس از اهدا، رفتار جنسی خارج از چارچوب خانواده، انجام تاتو و خالکوبی، حجامت و طب سوزنی، مداخلات پزشکی و مصرف مواد مخدر تزریقی بیشترین عوامل مؤثر بودند، که بیانگر ضرورت توجه بیشتر به این عوامل خطرساز در هنگام مشاوره و معاینه برای انتخاب اهداکننده است. در مطالعه صیقلی و همکاران در سالهای 2008-2007 در گروه اهداکنندگان مبتلا به عفونت HIV، عوامل خطرساز مصرف مواد مخدر تزریقی و رفتار جنسی پرخطر در مقایسه با گروه کنترل معنادار بودند.
در پژوهشی که برای شناسایی عوامل خطرساز در اهداکنندگان خون مبتلا به HCV توسط رضایی و همکاران در سالهای 2013-2009 انجام شد، همانند مطالعه صیقلی، مصرف مواد مخدر تزریقی مهمترین عامل و در رتبه بعد حجامت قرار داشت. رنجبر و همکاران در سالهای 2017-2015 با تغییراتی که در روش پژوهش انجام شد، اهداکنندگان دارای نتایج آزمایشهای مثبت تاییدی برای HCV را با پرسشنامه و دریافت نمونه برای انجام آزمایش مورد مطالعه قرار دادند. مهمترین عوامل خطرساز، مصرف مواد مخدر تزریقی و حجامت بودند (36-33). در این بررسی نیز همین عوامل خطرساز شایعترین علل اطلاعات پس از اهدا (PDI) بودند، که منجر به لوک بک واحدهای اهدایی گردیدند.
در بازه زمانی مطالعه، پاندمی کووید 19 رخ داد که عامل متغیر مهمی در تحلیل دادههای لوک بک، تعداد اهدا و حتی معافیت است، لذا توصیه میشود این مطالعه طی سالهای پس از پایان پاندمی کووید 19 (با توجه به اعلام WHO مبنی بر خاتمه پاندمی در نیمه اول سال 2023) نیز انجام شده و تغییرات تعداد واحدهای لوک بک با عدم تاثیر مهم پاندمی تحلیل شود.
نتیجهگیری
در این مطالعه تعداد واحدهای لوک بک به علت اطلاعات پس از اهدا در طی زمان سیر نزولی داشته و مهمترین اطلاعات پس از اهدا که منجر به لوک بک شده است شامل مصرف مواد مخدر و رفتار پر خطر جنسی خارج از خانواده با سیر کاهشی در طی 4 سال مطالعه و
تاتو و خالکوبی، انجام حجامت و طب سوزنی با روند افزایشی در این بازه زمانی است. در فرآیند انتخاب اهداکنندگان توجه بیشتر به این موارد که منجر به معافیت اهداکنندگان میشود، با هدف افزایش ضریب سلامت خون لازم است. برای تحلیل این تغییرات آمار اهدای خون، میزان معافیت و فراوانی نتایج مثبت آزمایشهای غربالگری
نیز ضروری است.
حمایت مالی
مطالعه فوق بدون حمایت مالی ارگان و نهاد خاصی انجام شده است.
ملاحظات اخلاقی
مطالعه حاضر دارای کد اخلاق IR.TMI.REC.1401.018 از کمیته اخلاق مؤسسه عالی آموزشی و پژوهشی طب انتقال خون تهران، ایران است.
عدم تعارض منافع
نویسندگان اظهار میکنند هیچگونه تعارض منافعی در این مطالعه وجود نداشته است.
نقش نویسندگان
دکتر صدیقه امینی کافیآبـاد: طراحـی مطالعـه، نگـارش و
ویرایش متن و راستیآزمایی اطلاعات
دکتر امیر تیمورپور: انجام تجزیه و تحلیل آماری، آمادهسازی جداول آماری و تهیه متن مرتبط با آنها
دکتر محمد حسام رفیعی: مطالعه و ویرایش مقاله
دکتر مهتاب مقصودلو: مشاوره در تجزیه و تحلیل آمار و نتایج، ویرایش مقاله
مینا مقتدایی: جمعآوری و راستیآزمایی اطلاعات و ویرایش مقاله
تشکر و قدردانی
بدینوسیله نویسندگان از همکاران مسئول لوک بک ادارات کل، ستاد و همکاران آزمایشگاههای غربالگری خونهای اهدایی تشکر و قدردانی مینمایند.
متن کامل: (607 مشاهده)
تحلیل روند واحدهای لوک بک بر مبنای اطلاعات پس از اهدای خون
در محمولههای ارسالی پلاسما برای پالایش قراردادی، 1400-1397
صدیقه امینی کافی آباد1 ، امیر تیمورپور2 ، محمد حسام رفیعی3 ، مینا مقتدایی4 ،
مهتاب مقصودلو5
1- متخصص آسیبشناسی ـ استاد مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
2- PhD آمار زیستی ـ استادیار مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
3- PhD بیوشیمی بالینی ـ استادیار مرکز تحقیقات انتقال خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
4- کارشناس ارشد انگلشناسی پزشکی ـ مرکز تحقیقات انتقال خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
5- متخصص پزشکی اجتماعی ـ استاد مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
در محمولههای ارسالی پلاسما برای پالایش قراردادی، 1400-1397
1- متخصص آسیبشناسی ـ استاد مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
2- PhD آمار زیستی ـ استادیار مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
3- PhD بیوشیمی بالینی ـ استادیار مرکز تحقیقات انتقال خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
4- کارشناس ارشد انگلشناسی پزشکی ـ مرکز تحقیقات انتقال خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
5- متخصص پزشکی اجتماعی ـ استاد مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون ـ تهران ـ ایران
 http://dx.doi.org/10.61186/bloodj.22.1.1 Citation: Amini-kafiabad S, Teimourpour A, Rafiee M.H, Moghtadaee M, Maghsudlu M. Analysis of Trends in Look-back Units Based on Post-Donation Information in Plasma Shipments for Contract Fractionation, 2018-2021. J Iran Blood Transfus. 2025: 22 (1) : 1-10. نویسنده مسئول: دکتر صدیقه امینی کافیآباد. استاد مرکز تحقیقات فرآوردههای بیولوژیک و سلامت خون ـ مؤسسه عالی آموزشی و پژوهشی طب انتقال خون، تهران، ایران صندوق پستی: 1157-14665 E-mail: s.amini@
کد اخلاق: IR.TMI.REC.1401.018 |
چکیده سابقه و هدف فرآیند لوک بک با هدف افزایش سلامت خون و فرآوردهها به عنوان یک اقدام اساسی، سال 1385 در سازمان انتقال خون ایران الزامی شد. بخش مهمی از آن برمبنای اطلاعات پس از اهدای خون بوده که متمرکز بر شناسایی رفتارهای پرخطر داوطلبان اهدا است که احتمالاً در فرآیند انتخاب اهداکننده شناسایی نشده است. در این مطالعه، روند تعداد واحدهای لوک بک مرتبط با رفتارهای پرخطر اهداکننده در پلاسمای مورد استفاده برای تولید داروهای مشتق از پلاسما بررسی میشود. مواد و روشها در این مطالعه مقطعی، اطلاعات لوک بک از مراکز انتقال خون مجاز به تأمین پلاسما برای تولید داروهای مشتق از پلاسما جمعآوری شد. دادهها شامل اطلاعات مربوط به واحدهای خون اهدایی، اهداکنندگان معاف، واحدهای پلاسما تعیین شده برای پالایش و واحدهای لوک بک مرتبط با اطلاعات پس از اهدای خون و همچنین عوامل خطرساز آنها بود. این اطلاعات بر اساس عوامل خطرساز مختلف طبقهبندی شدند. از آزمون کوکران- آرمیتاژ برای ارزیابی روند خطی خطر لوک بک در طول زمان استفاده شد. شاخص خطر نسبی به عنوان اندازه اثر، همراه با فاصله اطمینان 95% گزارش شد. تمام محاسبات آماری با استفاده از بسته 'epitools' در نرمافزار R انجام گردید. یافتهها از سال 1397 تا 1400، اهداکنندگان خون داوطلب و بدون چشمداشت، جمعاً 8035586 واحد خون اهدا کردند. 46% از واحدهای پلاسمای خونهای اهدایی به منظور تولید داروهای مشتق از پلاسما برای پالایشگرهای پلاسما ارسال شد. در بازه زمانی مطالعه، تعداد واحدهایی که به علت اطلاعات پس از اهدای خون نیاز به لوک بک داشتند از (2191) 4/24 به (1727) 7/17 به ازای هر ده هزار واحد پلاسمای ارسالی برای تولید دارو (001/0 p< ؛ 78/0-68/0 CI: 95% ؛ 73/0 RR:) کاهش یافت. مهمترین عوامل خطرساز که منجر به لوک بک برمبنای اطلاعات پس از اهدای خون شد شامل مصـرف مـواد مخـدر تزریقـی (001/0 p=) و رفتار جنسی پرخطر (001/0 p<) بود که در هر دو مورد کاهش معنادار داشت. در مقابل خالکوبی (01/0 p=) و فعالیتهای سنتی مانند حجامت و طب سوزنی (001/0 p<) روند افزایشی داشته و تغییرات معنادار بود. نتیجه گیری این مطالعه بر نقش حیاتی ارزیابی رفتارهای پرخطر شامل مواردی مانند مصرف تزریقی مواد مخدر، رفتارهای جنسی پرخطر و همچنین خالکوبی، حجامت و طب سوزنی در انتخاب اهداکنندگان خون برای افزایش سلامتی خون تأکید میکند. تقویت انتخاب اهداکنندگان میتواند تعداد واحدهای لوک بک را به حداقل برساند و نهایتاً ایمنی خون را بهبود بخشد. کلمات کلیدی: پلاسما، پالایش پلاسما، اهداکنندگان خون، سلامت خون |
مقدمه
با تأسیس سازمان انتقال خون در سال 1353، کیتهای غربالگری برای شناسایی آنتیژن سطحی ویروس هپاتیتB (HBsAg : Hepatitis B Surface Antigen) و سیفلیس برای سرویسهای انتقال خون در جهان در دسترس بود، لذا از ابتدای تأسیس سازمان در کشور، انجام آزمایش غربالگری برای این عوامل بیماریزا انجام گردید (1).
با شناسایی ویروس هپاتیت B در ابتدای دهه 1970 میلادی، با فاصله زمانی کوتاهی کیتهای غربالگری در اختیار مراکز انتقال خون جهان قرار گرفت (2). غربالگری ویروس نقص ایمنی انسانی-1 (HIV-1 : Human Immunodeficiency Virus-1) در سال 1368 و ویروس هپاتیتC (HCV : Hepatitis C Virus) در سال 1375 در سازمان انتقال خون آغاز شد (1). با تأسیس سازمان، اهدای خون به ازای دریافت پول ممنوع بوده که نشانه دیدگاه جامع، آیندهنگر و توجه ویژه به سلامت خون است.
با ظهور HIV-1 ، سرویسهای انتقال خون سراسر جهان، به انتخاب اهداکنندگان خون توجه بیشتری کرده و پرسشنامه مشاوره اهداکنندگان خون ارتقا یافت. سازمان جهانی بهداشت (WHO : World Health Organization) برای اولین بار با مشارکت جمعیت هلال احمر و صلیب سرخ در سال 1373، راهنمای انتخاب اهداکننده خون با توجه به ویروس نقص ایمنی انسانی را منتشر کرد (4، 3). در دهه اول قرن بیستم با انتشار جزوات متعددی در زمینه ساماندهی پرسشنامه انتخاب اهداکنندگان، حفظ سلامت واحدهای اهدایی خون و بیماران دریافتکننده همراه با حفظ سلامت اهداکنندگان مورد توجه قرار گرفت (5). بهرهبرداری از نرمافزارهای معتبر، تشکیل پرونده واحد برای هر اهداکننده با ثبت تمامی اطلاعات هر نوبت اهدا، سبب ارتقا سلامت خون گردید (1).
با شناسایی HIV با هدف جلوگیری از گسترش عفونت با آن از طریق بیمارانی که خون و فرآورده قبل از غربالگری برای HIV دریافت کرده بودند، در کشورهایی مانند کانادا، آمریکا و انگلیس لوک بک (Lookback) اجرایی شد. فرآوردههای تولیدی از خون اهداکنندگان مثبـت بـرایHIV قبـل از سـال 1980، شناسایــی شـده و
اطلاعات بیماران دریافتکننده استخراج گردید. این بیماران جهت انجام آزمایش برای تشخیص عفونت با HIV فراخوان شدند (8-6). علاوه بر HIV با کشف و شناسایی HCV ، لوک بک برای این ویروس نیز در کشورهای مختلفی مانند کانادا، دانمارک و آمریکا آغاز شد (11-9). در این فرآیند بیمارانی مانند کودکان، بیماران هموفیلی و بیماران مورد جراحیهای ویژه مانند جراحی قلب مورد آزمایش قرار میگرفتند تا در صورت ابتلا به عفونت با آموزش و درمانهای حمایتی از توسعه بیماری در جامعه جلوگیری شود (13، 12). این فرآیند را لوک بک (Lookback) نامگذاری و در استانداردهای انتقال خون مد نظر قرار دادند (15، 14).
در طی سالهای بعد لوک بک توسعه یافت و شامل واحدهای اهدایی بر اساس نتایج مثبت آزمایشهای غربالگری ویروسهای منتقله از راه خون یا اطلاعات پس از اهدا (Post donation information)، در رابطه با واحدهای اهدای قبلی اهداکننده بود. احتمال این که اهداکننده در دوره کمون بوده و با آزمایشهای غربالگری قابل شناسایی نباشد، بسیار اندک است، به همین دلیل انتقال عفونت پس از تزریق خون و فرآوردهها که به عنوان خطر باقیمانده (Residual Risk) نامیده میشود، ناچیز است. در این فرآیند واحدهای لوک بک امحا، قرنطینه، آزمایش واحدهای در دسترس و اطلاعرسانی به دریافتکنندگان انجام میشود (16، 8).
اطلاعات پس از اهدا (PDI) در چندین گروه قرار میگیرند که مهمترین اطلاعاتی هستند که توسط خود اهداکنندگان اعلام میشود که آن را به دو زیر گروه میتوان دستهبندی کرد. در یک دسته اهداکننده خود مطلع بوده و اعلام نکرده و اگر در حین مشاوره به پزشک اعلام میگردید، اهداکننده از اهدای خون توسط پزشک معاف میشد. گروه دیگر اهداکننده در زمان اهدا از عامل خطرساز مطلع نبوده و با اطلاع پس از اهدای خون، مرکز انتقال خون را مطلع میسازد و به عنوان Call-back شناخته میشود. عملکرد اهداکنندگان در این گروه انعکاس رفتار مسئولانه است هر چند خطر بالقوه برای سلامت خون باید مد نظر باشد. در انتقال خون و سلامت آن عدم افشای موارد منجر به معافیت در فرآیند انتخاب اهداکننده مهم است. شناسایی علت یا علل عدم اعلام رفتارهای پرخطر به پزشک و انجام اقدامات اصلاحی برای آنها میتواند منجر به ارتقا و بهبود فرآیند انتخاب اهداکنندگان و افزایش سلامت خون گردد (17).
با شروع پالایش قراردادی پلاسما در سال 1385 در کشور، فرآیند لوک بک با رویکرد نتایج آزمایشهای غربالگری استقرار یافت (18). متعاقباً "اطلاعات پس از اهدا" نیز اضافه شد. زیرگروه دیگر احتمال بیماری کروتسفلد ژاکوب (CJD : Creutzfeldt-Jakob)، را میتوان نام برد (20، 19، 13).
عواملی که در گروه رفتارهای پرخطر با عنوان اطلاعات پس از اهدا (PDI) بوده و منجر به لوک بک میشود، در جدول ذکر شده است (21، 15) (جدول 1). در این فرآیند امحا فرآوردههای خون از جمله پلاسما سبب افزایش ضایعات آن میشود.
ویلیامز و همکاران در سال 1997 با استفاده از پرسشنامه که در آن از رفتارهای پرخطر که میتواند سبب معافیت در آخرین اهدای خون اهداکنندگان شود، طرح پژوهشی اجرا کردند. بر اساس اطلاعات استخراجی از پرسشنامهها، 9/1% اهداکنندگان در زمان اهدای خون رفتار پرخطر داشته و در صورت اعلام آن از اهدای خون معاف میشدند. در این بررسی در 4/0% موارد در بازه زمانی 3 ماه قبل از اهدای خون رفتار پرخطر رخ داده بود (22). این مطالعه نیز دلیلی بر ضرورت انجام لوک بک به علت اطلاعات پس از اهدا را تایید میکند.
در این مطالعه واحدهای لوک بک به علت رفتار پرخطر در بازه زمانی 1400-1397 در محمولههای ارسالی پلاسما برای پالایش قراردادی که از مراکز انتقال خون به سردخانههای ستاد مرکزی سازمان حمل شده بود، از نظر تعداد و عوامل خطرساز تحلیل شدند تا در هنگام انتخاب اهداکنندگان عوامل خطرساز منجر به لوک بک مورد توجه بیشتر قرار گیرد.
مواد و روشها
در ایـن مطالعـه مقطعـی، آمار اهدای خون و معافیت از
اهدا از نرمافزار یکپارچه معتبر انتقال خون، نگاره به تفکیک سالهای مطالعه دریافت گردید. میزان ارسال پلاسما با استفاده از مکاتبات گواهی سلامت که سازمان انتقال خون به سازمان غذا و دارو و نمایندگان شرکتهای پالایشگر انجام میدهد، استخراج شد.
مراکز انتقال خون مورد مطالعه:
پالایش قراردادی پلاسما در سال 1385 با 15 پایگاه انتقال خون آغاز شد. در طی سالهای پس از آن با افزایش تعداد مراکزی که توسط حوزه تضمین کیفیت شرکتهای پالایشگر و نهادهای دولتی کشورهای شرکتهای پالایشگر بازرسی شدند، در سال 1400 به 28 مرکز افزایش یافت. در طی بازه زمانی مورد مطالعه 5 پایگاه انتقال خون بازرسی و مجوز ارسال پلاسما دریافت کردند.
جمعآوری اطلاعات لوک بک متعاقب اطلاعات پس از اهدا:
فرم لوک بک کاملاً خودکار توسط نرمافزار نگاره در ادارات کل آماده و چاپ شده، سپس توسط کارشناسانی که آموزشهای لازم را دریافت کردهاند، بررسی میگردد. در مرحله بعد توسط مدیریت/معاون اداره کل تأیید شده و در مرحله بعد به ستاد مرکزی سازمان انتقال خون ارسال میشود. اطلاعات این مطالعه از فرمهای لوک بک ارسالی از ادارات کل توسط کارشناسان ستاد استخراج گردید (18).
اطلاعاتی که در فرمهای لوک بک ثبت شده و در این مطالعه مورد استفاده قرار گرفت شامل شماره اهدا، تاریخ رخداد پرخطر، نوع رفتار پرخطر، تاریخ آخرین اهدا با نتایج آزمایشهای غربالگری منفی، تاریخ معافیت و نوع معافیت (دائمی یا موقت) و رفتار پرخطر طبق جدول یک بود.
دستهبندی رفتارهای پرخطر و نحوه شناسایی واحدهای لوک بک:
در موافقتنامه کیفی با شرکتهای پالایشگر و در سرویسهای انتقال خون کشورها، رفتارهای پرخطری که در حیطه لوک بک قرار میگیرد و بازههای زمانی که باید برگشت به عقب نمود، مشخص و تعیین شده و در جدول 1 ذکر شده است (21، 15). اگر تاریخ رفتار پرخطر در هنگام مصاحبه با اهداکننده توسط وی اعلام شود، تاریخ آخرین اهدای خون که در آزمایشهای غربالگری منفی گزارش شده شناسایی میگردد و از این تاریخ به مدت 183 روز تا رسیدن به تاریخ رفتار خطرساز، کلیه واحدهای اهدایی لوک بک میشود. این بازه زمانی حداکثر 6 ماه است و اگر رفتار پرخطر با واحد اهدایی منفی کمتر از 6 ماه فاصله داشته باشد، این بازه از 6 ماه کمتر خواهد بود. اگر اهداکننده تاریخ رفتار پر خطر را فراموش کرده باشد کلیه واحدهای پلاسما در بازه زمانی 6 ماه قبل از معافیت لوک بک خواهد شد.
روش تجزیه و تحلیل دادهها:
آمارهای توصیفی شامل میانگین و انحراف معیار به همراه فراوانی و درصد فراوانی گزارش شده است. به منظور بررسی روند تغییرات نسبت واحد لوک بک به تعداد واحد خون و واحد پلاسمای ارسالی از آزمون کوکران- آرمیتاژ استفاده شده است (23). از شاخص خطر نسبی به عنوان اندازه اثر برای مقایسه روند تغییرات لوک بک در طی زمان استفاده و گزارش شده است. در این مطالعه سطح معناداری 05/0 در نظر گرفته شده است. تمام محاسبات آماری توسط نرم افزار R و مقادیر خطر نسبی و فواصل اطمینان 95% آن به وسیله پکیج epitools محاسبه گردیده است.

یافتهها
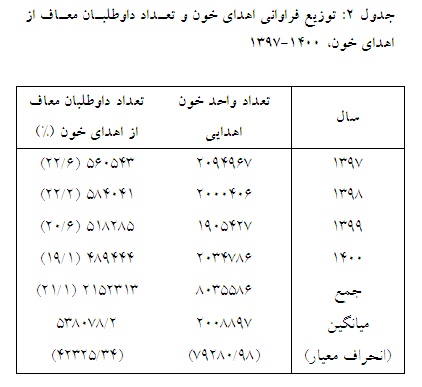
اهدای خون در طی سالهای 1397 تا 1400 در کشور به ازای هر سال تقریباً 2 میلیون واحد و میزان معافیت داوطلبان اهدای خون در محدوده 1/19% تا 6/22% بوده است (جدول 2).
روند کاهشی در تعداد واحدهای لوک بک به علل غیر مرتبط با نتایج آزمایشهای غربالگری در جدول مشاهده میشود (001/0 p<)(جدول 3). به طوری که تعداد آن در سال 1397 برابر 1/27 واحد در هر ده هزار (per ten thousand-ptt) واحد پلاسما ارسالی برای شرکتهای پالایشگر بوده که در سال 1400 این میزان به 9/18 واحد در ptt رسیده است (001/0 p< ؛ 74/0-66/0 :95%CI ؛ 69/0 RR:).
اهدای خون در طی سالهای 1397 تا 1400 در کشور به ازای هر سال تقریباً 2 میلیون واحد و میزان معافیت داوطلبان اهدای خون در محدوده 1/19% تا 6/22% بوده است (جدول 2).
روند کاهشی در تعداد واحدهای لوک بک به علل غیر مرتبط با نتایج آزمایشهای غربالگری در جدول مشاهده میشود (001/0 p<)(جدول 3). به طوری که تعداد آن در سال 1397 برابر 1/27 واحد در هر ده هزار (per ten thousand-ptt) واحد پلاسما ارسالی برای شرکتهای پالایشگر بوده که در سال 1400 این میزان به 9/18 واحد در ptt رسیده است (001/0 p< ؛ 74/0-66/0 :95%CI ؛ 69/0 RR:).



مقادیر خطر نسبی لوک بک غیر از موارد لوک بک به علت نتایج آزمایشهای غربالگری تکرارپذیر در هر سال در مقایسه با سال قبل در جدول 3 گزارش شده است. روند کاهشی در میزان تعداد واحد لوک بک به علت اطلاعات پس از اهدا مبتنی بر رفتارهای پر خطر در طی زمان از 1397 تا 1400 نسبت به میزان واحد پلاسمای ارسالی مشاهده میشود (001/0 p<)، در سال اول مطالعه نسبت واحد لوک بک به علت رفتارهای پر خطر 4/24 در ده هزار واحد پلاسمای ارسالی است که در سال آخر مطالعه به 7/17 در ده هزار واحد پلاسمای ارسالی کاهش یافته است (001/0 p< ؛ 78/0-68/0 :95%CI ؛ 73/0 RR:)، مقادیر خطر نسبی واحد لوک بک به علت رفتارهای پر خطر در هر سال در مقایسه با سال قبل و اطلاعات مرتبط با فراوانی تعداد واحد پلاسمای ارسالی و تعداد واحد لوک بک به علت رفتارهای پرخطر در هر سال در جدول 3 گزارش شده است.
طبق اطلاعات جدول 3، با توجه به اطلاعات ستونهای تعداد واحدهای لوک بک به علل غیر مرتبط با نتایج آزمایشهای غربالگری و تعداد واحدهای لوک بک به علت رفتارهای پرخطر، تعداد واحدهای لوک بک غیر مرتبط با اطلاعات پس از اهدا و نتایج آزمایشهای غربالگری (مانند CJD و سایر علل) نیز سیر نزولی داشته و از 7/2 به 2/1 به ازای هر ده هزار واحد، معادل 6/1 واحد در هر ده هزار واحد پلاسما ارسالی کاهش نشان میدهد.
اطلاعات پس از اهدا، یا رفتارهای پرخطر که منجر به لوک بک شدند در 15 گروه طبق موافقت نامه کیفی با پالایشگرها قرار گرفتند. در 5 گروه در بازه زمانی مورد مطالعه نمونهای گزارش نشده است. در بین 10 گروه دیگر بیشترین فراوانی در گروههای رفتار جنسی خارج از چارچوب خانواده، حجامت و طب سوزنی، انجام تاتو و خالکوبی، آندوسکوپی و مصرف مواد مخدر تزریقی با فراوانی 6/5% تا 3/39% گزارش شد. از این 5 گروه، 4 گروه شامل رفتار جنسی خارج از چارچوب خانواده (001/0 p<)، حجامت و طب سوزنی (001/0 p<)، انجام تاتو و خالکوبی (01/0 p=) و مصرف مواد مخدر تزریقــی
(001/0 p=) در بازه زمانی مطالعه با یک روند معنادار گزارش شده است.
بحث
در طی سالهای 1400-1397 بخشی از واحدهای لوک بک که مرتبط با انتخاب اهداکننده و رفتارهای پرخطر است، روند کاهشی نشان میدهد. شاید به نظر برسد این کاهش به نحوه انتخاب اهداکننده و بهبود آن مرتبط باشد اما از سوی دیگر شاهد روند کاهشی تعداد معافیت هستیم که با توجه به پاندمی کووید-19 و افزایش حداقل 4 سؤال به پرسشنامه اهداکنندگان برای جلوگیری از اهدای خون در داوطلبانی که احتمال انتقال کووید 19 را دارند، نیاز به توجه بیشتری در تحلیل یافتهها دارد (24). در سال 1399 کاهش آمار اهدای خون به دنبال کاهش داوطلبان برای اهدای خون مشاهده میگردد. دربازه زمانی این مطالعه، نوسان در فراوانی موارد مثبت HBV ، HCVوHIV در کشور گزارش شده است از جمله فراوانی HBV و HCV در سال 2020 نسبت به سال 2019 در جمعیت اهداکنندگان افزایش یافته و این افزایش معنادار گزارش شده است (27-25). با توجه به موارد فوق، اعلام نظر برای بهبود و ارتقای فرآیند انتخاب اهداکننده همانند سایر کشورها در این بازه زمانی که مصادف با پاندمی کووید بوده، با چالشهای جدی روبرو است (29-27).
در سال 1399به عنوان اولین سال پاندمی کووید 19 که برای جامعه جهانی غیرمترقبه و بدون آمادگیهای لازم برای مراکز انتقال خون در برخورد با این پاندمی بود، کمترین تعداد لوک بک به علت رفتارهای پرخطر، 1392 مورد به ازای هر 10000 واحد پلاسما ارسالی، گزارش شده است. اما در سال 1400 این تعداد به 1774 واحد به ازای هر 10000 واحد پلاسما ارسالی برای پالایش قراردادی رسیده است. این تغییرات همراه با تغییر میزان معافیت از اهدای خون در سال 1400 همانند سایر کشورهای جهان میتواند متأثر از پاندمی کووید 19 باشد (32-30).
از عوامل خطر ساز در لوک بک به دنبال اطلاعات پس از اهدا، رفتار جنسی خارج از چارچوب خانواده، انجام تاتو و خالکوبی، حجامت و طب سوزنی، مداخلات پزشکی و مصرف مواد مخدر تزریقی بیشترین عوامل مؤثر بودند، که بیانگر ضرورت توجه بیشتر به این عوامل خطرساز در هنگام مشاوره و معاینه برای انتخاب اهداکننده است. در مطالعه صیقلی و همکاران در سالهای 2008-2007 در گروه اهداکنندگان مبتلا به عفونت HIV، عوامل خطرساز مصرف مواد مخدر تزریقی و رفتار جنسی پرخطر در مقایسه با گروه کنترل معنادار بودند.
در پژوهشی که برای شناسایی عوامل خطرساز در اهداکنندگان خون مبتلا به HCV توسط رضایی و همکاران در سالهای 2013-2009 انجام شد، همانند مطالعه صیقلی، مصرف مواد مخدر تزریقی مهمترین عامل و در رتبه بعد حجامت قرار داشت. رنجبر و همکاران در سالهای 2017-2015 با تغییراتی که در روش پژوهش انجام شد، اهداکنندگان دارای نتایج آزمایشهای مثبت تاییدی برای HCV را با پرسشنامه و دریافت نمونه برای انجام آزمایش مورد مطالعه قرار دادند. مهمترین عوامل خطرساز، مصرف مواد مخدر تزریقی و حجامت بودند (36-33). در این بررسی نیز همین عوامل خطرساز شایعترین علل اطلاعات پس از اهدا (PDI) بودند، که منجر به لوک بک واحدهای اهدایی گردیدند.
در بازه زمانی مطالعه، پاندمی کووید 19 رخ داد که عامل متغیر مهمی در تحلیل دادههای لوک بک، تعداد اهدا و حتی معافیت است، لذا توصیه میشود این مطالعه طی سالهای پس از پایان پاندمی کووید 19 (با توجه به اعلام WHO مبنی بر خاتمه پاندمی در نیمه اول سال 2023) نیز انجام شده و تغییرات تعداد واحدهای لوک بک با عدم تاثیر مهم پاندمی تحلیل شود.
نتیجهگیری
در این مطالعه تعداد واحدهای لوک بک به علت اطلاعات پس از اهدا در طی زمان سیر نزولی داشته و مهمترین اطلاعات پس از اهدا که منجر به لوک بک شده است شامل مصرف مواد مخدر و رفتار پر خطر جنسی خارج از خانواده با سیر کاهشی در طی 4 سال مطالعه و
تاتو و خالکوبی، انجام حجامت و طب سوزنی با روند افزایشی در این بازه زمانی است. در فرآیند انتخاب اهداکنندگان توجه بیشتر به این موارد که منجر به معافیت اهداکنندگان میشود، با هدف افزایش ضریب سلامت خون لازم است. برای تحلیل این تغییرات آمار اهدای خون، میزان معافیت و فراوانی نتایج مثبت آزمایشهای غربالگری
نیز ضروری است.
حمایت مالی
مطالعه فوق بدون حمایت مالی ارگان و نهاد خاصی انجام شده است.
ملاحظات اخلاقی
مطالعه حاضر دارای کد اخلاق IR.TMI.REC.1401.018 از کمیته اخلاق مؤسسه عالی آموزشی و پژوهشی طب انتقال خون تهران، ایران است.
عدم تعارض منافع
نویسندگان اظهار میکنند هیچگونه تعارض منافعی در این مطالعه وجود نداشته است.
نقش نویسندگان
دکتر صدیقه امینی کافیآبـاد: طراحـی مطالعـه، نگـارش و
ویرایش متن و راستیآزمایی اطلاعات
دکتر امیر تیمورپور: انجام تجزیه و تحلیل آماری، آمادهسازی جداول آماری و تهیه متن مرتبط با آنها
دکتر محمد حسام رفیعی: مطالعه و ویرایش مقاله
دکتر مهتاب مقصودلو: مشاوره در تجزیه و تحلیل آمار و نتایج، ویرایش مقاله
مینا مقتدایی: جمعآوری و راستیآزمایی اطلاعات و ویرایش مقاله
تشکر و قدردانی
بدینوسیله نویسندگان از همکاران مسئول لوک بک ادارات کل، ستاد و همکاران آزمایشگاههای غربالگری خونهای اهدایی تشکر و قدردانی مینمایند.
نوع مطالعه: پژوهشي |
موضوع مقاله:
انتقال خون
فهرست منابع
1. Amini Kafi-abad S, Rezvan H, Abolghasemi H, Talebian A. Prevalence and trends of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus among blood donors in Iran, 2004 through 2007. Transfusion 2009; 49(10): 2214-20. [DOI:10.1111/j.1537-2995.2009.02245.x] [PMID]
2. Cohn Claudia S, Delaney M, Johnson ST, Louis MK. Technical Manual. 21st ed. USA: AABB; 2023. p. 169-214.
3. WHO. Blood donor counselling: implementation guidelines. USA: WHO; 2014.
4. WHO. Blood donor selection: guidelines on assessing donor suitability for blood donation. USA: WHO; 2012.
5. WHO. Guidelines for blood donor counselling on human immunodeficiency virus (HIV). USA: WHO; 1994.
6. Byrne L, Brant LJ, Davison K, Hewitt P. Transfusion transmitted human immunodeficiency virus (HIV) from seroconverting donors is rare in England and Wales: results from HIV lookback, October 1995 through December 2008. Transfusion 2011; 51(6): 1339-45. [DOI:10.1111/j.1537-2995.2010.02996.x] [PMID]
7. Gill M, Towns D, Allaire S, Meyers G. Transmission of human immunodeficiency virus through blood transfusion: the use of lookback and traceback approaches to optimize recipient identification in a regional population. Transfusion 1997; 37(5): 513-6. [DOI:10.1046/j.1537-2995.1997.37597293883.x] [PMID]
8. FDA. Nucleic Acid Testing (NAT) for Human Immunodeficiency Virus Type 1 (HIV-1) and Hepatitis C Virus (HCV): Testing, Product Disposition, and Donor Deferral and Reentry. USA: Department of Health and Human Sciences; 2017.
9. FDA. Guidance for Industry: "Lookback" for Hepatitis C Virus HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test Results Indicating Infection with HCV. USA: Department of Health and Human Sciences; 2013.
10. Pawson R, Rajan S, Hazlehurst G, Dusheiko G, Miller R, Hewitt P, et al. Hepatitis C lookback programme: a single hospital experience. Transfus Med 1999; 9(3): 189-93. [DOI:10.1046/j.1365-3148.1999.00197.x] [PMID]
11. Christensen PB, Groenbæk K, Krarup HB, Gaub J, Møller A, Skinhøj P, et al. Transfusion-acquired hepatitis C: the Danish lookback experience. Transfusion 1999; 39(2): 188-93. [DOI:10.1046/j.1537-2995.1999.39299154734.x] [PMID]
12. Tynell E. Prevention of transfusion transmitted infections. Donor screening and characteristics of recipient populations [Thesis]. Stockholm: Karolinska University; 2005. p. 29-30.
13. Menozzi D, Udulutch T, Llosa AE, Galel SA. HCV lookback in the United States: effectiveness of an extended lookback program. Transfusion 2000; 40(11): 1393-8. [DOI:10.1046/j.1537-2995.2000.40111393.x] [PMID]
14. IBTO. Iranian National Standards for Blood Transfusion. 3rd ed. Tehran: Teimourzadeh Pub; 2016. p. 33. [Persian]
15. JPAC. Chapter 5.7: Whole blood donation. Guidelines for the Blood Transfusion and Tissue Transplantation Services in the UK. https://www. transfusionguidelines. org. Accessed 24 October 2024.
16. Health Canada. Guidance Document: Blood Regulations. Canada: Health Canada; 2016. p. 242.
17. Vuk T, Garraud O, Politis C. Post-donation information management. Transfus Clin Biol 2021; 28(4): 407-13. [DOI:10.1016/j.tracli.2021.08.006] [PMID]
18. Teimourpour A, Moghtadaee M, Amini-Kafiabad S, Maghsoodlu M, Rafiee M. Investigating the trend of look-back units in the plasma for contract fractionation during 2018-2021. Sci J Iran Blood Transfus Organ 2024; 21(3): 185-96. [Article in Farsi]
19. FDA. Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products. USA: Department of Health and Human Sciences; 2020.
20. Kiely P, Hoad VC, Wood EM. False positive viral marker results in blood donors and their unintended consequences. Vox Sang 2018; 113(6): 530-9. [DOI:10.1111/vox.12675] [PMID]
21. Ramsey G. Blood component recalls and market withdrawals: frequency, reasons, and management in the United States. Transfus Med Rev 2013; 27(2): 82-90. [DOI:10.1016/j.tmrv.2012.11.001] [PMID] []
22. Williams AE, Thomson RA, Schreiber GB, Watanabe K, Bethel J, Lo A, et al. Estimates of infectious disease risk factors in US blood donors. JAMA 1997; 277(12): 967-72. [DOI:10.1001/jama.1997.03540360035027]
23. Agresti A. Categorical Data Analysis. USA: John Wiley & Sons; 2018. p. 46.
24. Rafiee MH, Kafiabad SA, Maghsudlu M. Analysis of blood donors' characteristics and deferrals related to COVID-19 in Iran. Transfus Apher Sci 2021; 60(2): 103049. [DOI:10.1016/j.transci.2020.103049] [PMID] []
25. Iranian Blood Transfusion Organization's Booklet; 2023. Available from: https://en.ibto.ir/uploads/En-ketabche1-final.pdf.
26. Ranjbar Kermani F, Chegini A, Sharifi Sh, Zadsar M. Changing Blood Donor's Characteristics after COVID-19 Emergence, the Young and Female Blood Donors' Role to Maintain A Safe Supply. Iranian Red Crescent Medical Journal (IRCMJ) 2024; 26(1): 1-7.
27. Divkolaye N, Arabkhazaeli A, Hajibeigi B, Eshghi P. The impact of COVID-19 on blood safety and availability in the Islamic Republic of Iran. East Mediterr Health J 2022; 28(11): 823-8. [DOI:10.26719/emhj.22.080] [PMID]
28. Conti G, Notari IV EP, Dodd RY, Kessler D, Custer B, Bruhn R, et al. Changes in transfusion-transmissible infection prevalence and demographics among US blood donors during the COVID-19 pandemic. Transfusion 2024; 64(6): 1040-9. [DOI:10.1111/trf.17851] [PMID]
29. Burananayok S, Nachatri W, Choothanorm P, Kusolthammarat K, Jaruthamsophon K, Yodsawad C, et al. COVID-19 impact on blood donor characteristics and seroprevalence of transfusion-transmitted infections in southern Thailand between 2018 and 2022. Sci Rep 2024; 14(1): 7920. [DOI:10.1038/s41598-024-57584-z] [PMID] []
30. Loua A, Kasilo OMJ, Nikiema JB, Sougou AS, Kniazkov S, Annan EA. Impact of the COVID-19 pandemic on blood supply and demand in the WHO African region. Vox Sang 2021; 116(7): 774-84. [DOI:10.1111/vox.13071] [PMID] []
31. Yazer MH, Jackson B, Pagano M, Rahimi-Levene N, Peer V, Bueno JL, et al. Vox Sanguinis international forum on transfusion services' response to COVID-19: summary. Vox Sang 2020; 115(6): 536. [DOI:10.1111/vox.12943] [PMID] []
32. Franchini M, Farrugia A, Velati C, Zanetti A, Romanò L, Grazzini G, et al. The impact of the SARS-CoV-2 outbreak on the safety and availability of blood transfusions in Italy. Vox Sang 2020; 115(8): 603. [DOI:10.1111/vox.12928] [PMID] []
33. Seighali F, Divkolaye NSH, Rezaei N, Kangarloo M. Risk factors associated with human immunodeficiency virus infection in blood donors in Iran: A case-control study. Asian J Transfus Sci 2021; 15(2): 183-8. [DOI:10.4103/ajts.AJTS_47_18] [PMID] []
34. Rezaei N, Amini-Kafiabad S, Maghsudlu M, Abolghasemi H. Risk factor analysis of hepatitis C virus seropositivity in Iranian blood donors: a case-control study. Transfusion 2016; 56(7): 1891-8. [DOI:10.1111/trf.13660] [PMID]
35. Ranjbar Kermani F, Hosseini KM, Kafi-abad SA, Maghsudlu M, Sharifi Z, Mansournia MA, et al. Update on transmission modes of hepatitis C virus among volunteer Iranian blood donors: analysis of a matched case-control study by penalized conditional logistic regression. Hepat Mon 2018; 18(10): e69395. [DOI:10.5812/hepatmon.69395]
36. Lieshout-Krikke RW, van 't Ende EA, Slot E, Karomi S, Kivit RM, Zaaijer HL. Infectivity of pre-seroconversion donations: an analysis of lookback exercises in The Netherlands, 2000-2006. Vox Sang 2012; 102(3): 193-7. [DOI:10.1111/j.1423-0410.2011.01537.x] [PMID]
ارسال پیام به نویسنده مسئول
| بازنشر اطلاعات | |
 |
این مقاله تحت شرایط Creative Commons Attribution-NonCommercial 4.0 International License قابل بازنشر است. |







